Spotlight on Akiva Mintz, MD, PhD – Columbia University
– Bridging the “valley of death” in translational medicine –
 Prof. Akiva Mintz, M.D., Ph.D., is a physician-scientist whose translational research focuses on developing cancer therapies with non-invasive molecular imaging.
Prof. Akiva Mintz, M.D., Ph.D., is a physician-scientist whose translational research focuses on developing cancer therapies with non-invasive molecular imaging.
Dr. Mintz is professor of radiology at Columbia University Irving Medical Center (CUIMC) and attending radiologist at New York-Presbyterian Hospital. He also is vice chair of translational research, director of the Columbia University PET Center and chief of the nuclear medicine division.
With over 20 years of experience in translational cancer research, Dr. Mintz has developed anticancer therapies with over 100 publications, presentations and patents.
A key goal of your Translational Therapeutics Accelerator (TRx) program at CUIMC is to accelerate translation of novel medical discoveries from the lab to the clinic by positioning the technology for commercialization. What are in your opinion the requirements to successfully bridge the “Translational Valley of Death”?
I believe that preclinical animal imaging equipment with similar functionality to what can be achieved with clinical human scanners can expedite drug delivery. Successful preclinical imaging programs can determine how to derive the most important, biologically meaningful information from an imaging study so that treatments can shift from a qualitative to quantitative science. However, sometimes failures are not so much caused by factors in the domains of biology, imaging and “omics” themselves, but rather in the other areas, such as lack of commercialization training or funding of key experiments. At Columbia, the Translational Therapeutics Accelerator was launched in 2016 to act as a centralized resource fostering collaboration throughout the Columbia campuses and is a partnership between the Irving Institute for Clinical and Translational Research, Columbia Technology Ventures, Columbia University Irving Medical Center and other biomedical accelerators. This close collaboration helps academic translational research projects move beyond the valley of death to reach commercialization milestones and clinical implementation.
In your more recent work, you have focused on developing strategies for delivering diagnostics and therapies to the brain by crossing or bypassing the blood–brain barrier (BBB). Any reason for focusing on one of the more difficult challenges in drug delivery?
Drug traversal across the blood-brain barrier has come under increasing scrutiny recently, particularly because most therapies and medicines are limited due to their inability to cross this barrier, hence reducing treatment options for maladies affecting the brain. Therefore, we are developing novel techniques to deliver anti-cancer drugs and use advanced molecular imaging to visualize real-time drug delivery. This allows us to expand options to patients for hard-to-reach tumors, such as brain cancer. We have built a complete small animal neurosurgery and imaging suite and acquired a stereotactic small animal HIFU device to support our NIH-funded projects that deliver therapeutics and visualize real-time drug delivery using advanced multimodality molecular imaging. 3D optical imaging(OI)/CT has been a great preclinical tool for visualizing BBB barrier permeability and drug delivery, which we validated against translational PET methods.
In a recent publication, you concluded that the use of 3D OI/CT for spatial reference of locoregionally delivered drugs, cells, and HIFU-mediated BBB permeability creates the possibility for its use as a preclinical tool for drug development.
Indeed, we demonstrated that we can use 3D OI/CT to directly label a prototype antibody therapy with Cy7 and
image its distribution after HIFU-mediated BBB disruption as a monitoring tool to measure the
delivery of therapeutic proteins to the tumors through the permeabilized BBB. The Cy7 labeled antibody distribution was compared to 89Zr immunoPET (see figure). This is highly translational when testing the preclinical efficacy of potential macromolecular therapeutic strategies such as immunotherapy. Importantly, 3D OI/CT can act as an accessible preclinical surrogate for using radiolabeled entities in future clinical testing. For further information, click here
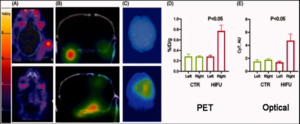
Systemic therapeutic delivery through HIFU induced BBB permeability using PET/CT and optical/CT. Control mice were not exposed to HIFU (top panels). (A) 30 min PET/CT images obtained 24 h p.i. shown in axial projection. (B) 3D optical/CT imaging to detect intracranial Cy7-anti-PD1 antibody accumulation, shown in coronal projection. (C) Brains were dissected and both PET and optical images were taken. (D) and (E): PET & Optical uptake were quantitated & analyzed for statistical significance. (Ó Drug Deliv., 2020 Dec;27(1): 1686-1694)
Concurrent in-vivo multimodal imaging where image sets of the same animal can be acquired simultaneously, or sufficiently close in time, to produce a combined enhanced image seems to be a useful tool to bridge the gap between preclinical research and clinical implementation. Do you agree?
Yes, there are numerous advantages to concurrent multimodal imaging. As illustrated for brain imaging, we also have conducted 3D OI/CT imaging for quantifying the abnormal epithelial permeability that is seen in lung pathologies, such as ARDS (acute respiratory distress syndrome) or COVID-19. The ability to use Cy7-albumin 3D OI/CT imaging as a preclinical translational surrogate for ⁶⁸Ga-albumin offers an accessible high throughput means to rapidly screen potential therapeutics against lung diseases that clinically manifest with endothelial permeability. Furthermore, combining optical and nuclear imaging technologies on a single stage can serve as an effective translational platform between the very widely used optical techniques used in small animal models, and nuclear medicine radiotracer assays that can be moved from mice to humans. For further reading, click here.
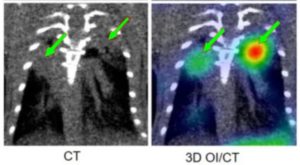 Coronal images of Cy7-albumin uptake in the lungs of lipopolysaccharide (LPS) induced lung injury in a mouse model for ARDS.
Coronal images of Cy7-albumin uptake in the lungs of lipopolysaccharide (LPS) induced lung injury in a mouse model for ARDS.
Note that Cy7-albumin infiltrates visible on CT (yellow arrows) are co-localized for quantitation with 3D tomographic OI/CT (right panel).
(Ó Molecular Biomedicine volume 1, Article number: 17, 2020)
We assume that this applies to molecular targeted radiotherapies as well. Optical and PET markers could eventually be used as surrogate markers before concurrent imaging with high-energy SPECT of alpha- and beta-emitting radiotherapy isotopes?
I agree that the use of surrogate diagnostic optical or nuclear markers are a critical part of molecular targeted radiotherapy workflow. Especially, alpha-particles have very promising potential as “magic cancer bullets” due to their much higher potency per delivered particle (100 to 500-fold) compared to β-particles. Furthermore, their very short range makes them more precise compared to β-particles, which have a much longer range. However, in some cases this precision may be a drawback because if there are tumor cells nearby that do not have the targeted biomarker, the α-particles will not reach them. Moreover, we have learned that cancer is more of a collection of diseases, with every patient having a different variety that will likely have to be treated with different therapies. The most exciting technological advances are leading us to an era of precision personalized medicine, where each patient’s cancer is analyzed for its particular drivers and vulnerabilities using advanced diagnostic approaches including concurrent multimodal tomographic imaging with Optical or PET reporters. This information that will allow oncologists to choose from a collection of therapies that will have the best chance of curing that particular cancer.
