Spotlight on Prof. Marion de Jong – Erasmus MC Rotterdam
On the frontline of fighting cancer with theranostic tracers
As one of the trailblazers in theranostic imaging, Marion de Jong, Ph.D., heads the TRACER group at the renowned Erasmus MC in Rotterdam, the Netherlands. She leads the department of development of preclinical and translational evaluation of new probes for theranostic applications.
 Prof. de Jong is co-author of over 400 peer-reviewed papers, is the principal investigator in several national and international research projects and has served in multiple international committees and boards. She is also credited with inventive contributions to the launch of 177Lu-Luthera, the first FDA and EMA approved peptide receptor radionuclide therapy (PRRT) to combat neuroendocrine tumors (NETs).
Prof. de Jong is co-author of over 400 peer-reviewed papers, is the principal investigator in several national and international research projects and has served in multiple international committees and boards. She is also credited with inventive contributions to the launch of 177Lu-Luthera, the first FDA and EMA approved peptide receptor radionuclide therapy (PRRT) to combat neuroendocrine tumors (NETs).
With over 25 years of experience with the preclinical and clinical evolution and development of the first successful theranostic radionuclide approach, are there any lessons you would like to share?
Initially, we treated test animals and patients with high doses of 111In-pentetreotide, a very popular diagnostic agent known as OctreoScan. After several years of PRRT experience with 111In as Auger- electron emitter for radionuclide therapy, it became clear that other radionuclides, such as 90Y or a new radionuclide 177Lu, could be better suited for a therapeutic regimen, due to longer range of tissue penetration. Unfortunately, 90Y has a rather poor image resolution and is not well suited for preclinical evaluation. Moreover, it became obvious that the novel analog Tyr3-octreotate was superior to other somatostatin analogs regarding the receptor affinity and also it became clear that measures to protect against renal failure by uptake of the radionuclide would be needed. That is the reason we started preclinical and clinical investigations to optimize kidney protection with the amino acids lysine and arginine during PRRT with somatostatin analogs, and our lysine–arginine formulation as well as the use of 177Lu is still used worldwide today.
We then introduced several new and exciting tracers (mostly radiopeptides and novel radionuclides) into the (pre)clinic for imaging and radionuclide therapy of e.g. neuroendocrine tumors, exocrine pancreatic tumors, melanoma, prostate- and breast cancer, and medullary thyroid cancer.
Multidisciplinary work in this field is key and this might mean blood, sweat, and tears, but also you will certainly have big fun together. This because of the very good collaborations that can be built in the field with added value for all partners, both researchers in academic centers and industry.
Go for new developments, there are so many opportunities ahead, theranostics are the future.
Also, invest and love your youngsters in the team; education is an important part of the research.
Can you give your perspective on future radionuclide therapy improvements?
Many efforts have been made to increase the in-vivo stability of radiopeptides by introducing structural modifications. In a collaboration with Dr. B. Nock and Dr. T. Maina and their team, we showed that co-administration of compounds that inhibit the activity of certain enzymes along with a therapeutic or diagnostic compound, in particular a peptide-radioligand, significantly enhanced in-vivo targeting. For instance, we demonstrated that co-injection of a neutral endopeptidase inhibitor can stabilize radiolabeled bombesin, minigastrin, neurotensin, and somatostatin analogs in vivo, leading to enhanced tumor uptake and improved tumor visualization.
In this respect, preclinical multimodality systems that enable in-vivo imaging with high resolution in mouse models such as the MILabs VECTor have proven to be highly valuable for such discoveries. Within-subject valuation of two treatment conditions, co-registered in space and time, can save quite some development time by improving statistical analysis as well as potentially reducing the number of required animals (see also below)
Alpha emitters are very interesting for radionuclide therapy, as they are powerful and emit particles with short particle ranges, limiting toxicity to healthy tissues and organs (see also below).
As we need to know what we are doing during radionuclide therapy, we need to visualize not only multicellular but also (sub) cellular processes, using imaging of DNA damage and repair processes and target (distribution) at the (sub)cellular level.
Are there preclinical system enhancements that may further advance theranostic developments?
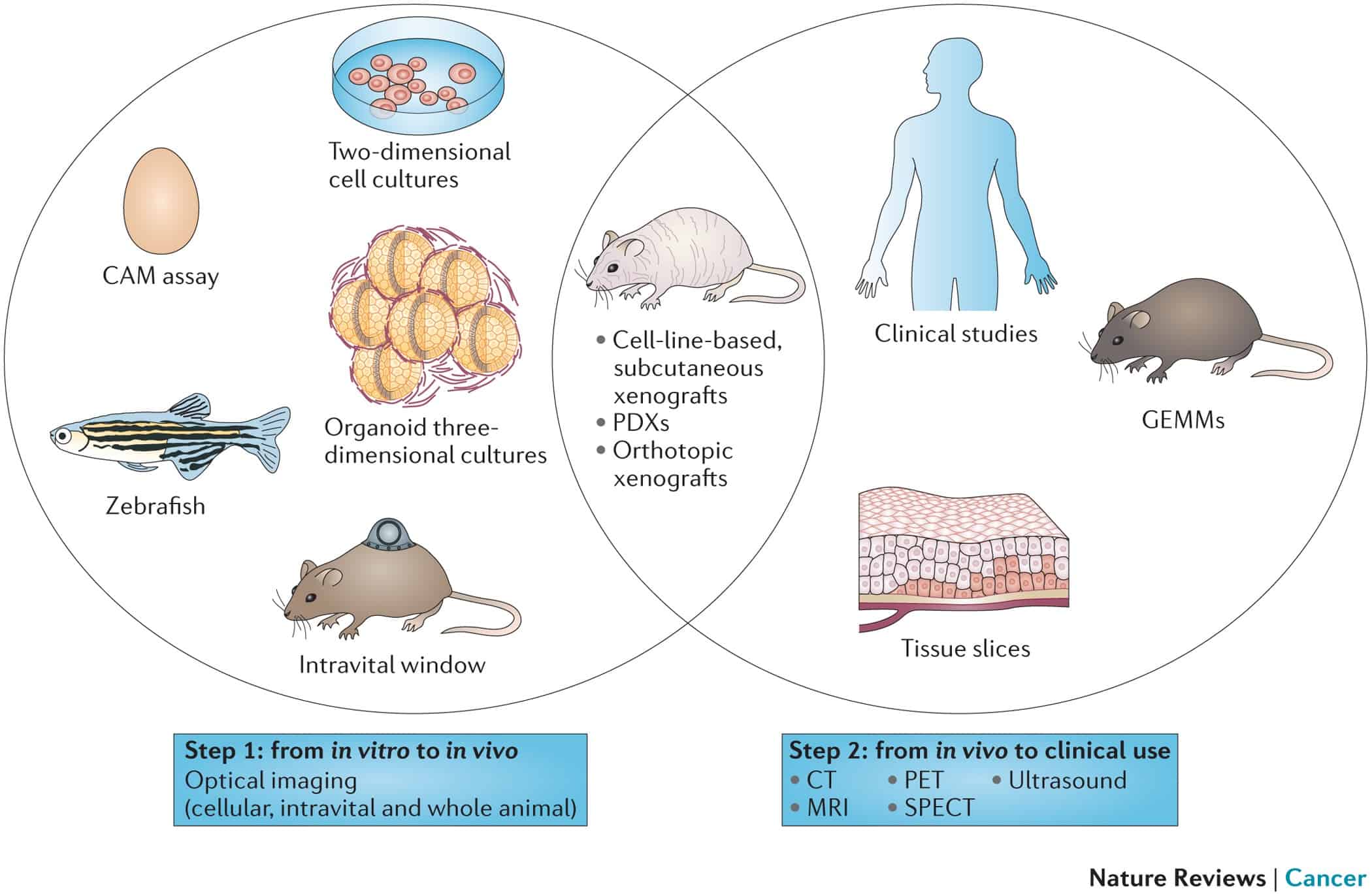
As illustrated in our publication: “Imaging Preclinical Tumour Models: Improving Translational Power.” Nature Reviews Cancer 14, no. 7 (July 2014), preclinical imaging plays an important role in the cycle of development and evaluation of new radiolabeled probes for theranostic applications.
Typically, two steps in the translation of in vitro results to clinical practice can be distinguished. The first step comprises translation from in vitro research to analysis in preclinical animal models, whereas the second step involves translation from animal models to the clinic.
We use MILabs’ multimodal imaging modalities in the second step, in model systems that are suitable for whole-animal in vivo optical imaging include subcutaneous and orthotopic cell-line-based or patient-derived xenograft models (PDXs), as well as genetically engineered mouse models (GEMMs). These different tumor models can be used in the second step, from animal model to clinic, and analyzed with clinically applied imaging modalities such as positron emission tomography (PET), single-photon emission computed tomography (SPECT), computed tomography (CT), magnetic resonance imaging (MRI) and ultrasound; all of which are adapted for small-animal imaging. In addition, patient tissue-slice systems and early phase clinical studies will further bridge the gap between the laboratory and clinical practice.
MILabs’ recent system additions to perform quantitative ex-vivo 3D autoradiography and optical imaging offer interesting enhancements to the nuclear medicine techniques (See e.g. “Molecular imaging: the emerging role of optical imaging in nuclear medicine”). The advantages of optical probes include the fact that they not use radioactivity, thus reducing the risk for side effects, plus the ability to directly translate fluorescence technologies available for molecular cell biology applications to the in vivo situation: An important development in the field of radiopeptides involving optical probes has been the introduction of hybrid derivatives, containing both a fluorescent and a radioactive label, as these have significant implications in the field of imaging-guided surgery.
The introduction of Powerful α-Emitters
I believe that the ability of MILabs VECTor system for in-vivo imaging of α-emitters at sub-mm resolution represents an important development in the field of radionuclide therapy. After completing successful preclinical studies, a first-in-human study showed that 213Bi-DOTATOC can eradicate neuroendocrine liver metastases pretreated with 177Lu-DOTATOC. Non-operable and critically located gliomas have been treated with α-targeted therapy as well, by local injection of 213Bi-DOTA-substance P, providing local irradiation of the tumor. The short tissue range of 213Bi prevents damage to adjacent brain areas. Up till now, this therapy has proven to be feasible and safe, with only mild adverse effects observed. Despite the promising preliminary results obtained with 213Bi-DOTATOC and 213Bi-substance P in patients, the short half-life of 213Bi (45.6 min) and the costs and limited availability of 225Ac/213Bi generators limit clinical use. However, the accelerator-driven production of 225Ac may allow it to be produced in quantities sufficient to supply centers for clinical trials and make the treatment available to a wider range of institutes. Moreover, since the MILabs system is suited to image a wide range of α-emitters, including 212Pb, 209At/211At, 221Fr, 223Ra may open new avenues for improved radionuclide therapy applications.
Can you elaborate on your further expectations & future directions?
We currently work on the introduction of several new and exciting tracers as mentioned above. Moreover, we currently expand our work on combination therapies and collaborate on combinations with e.g. epigenetic drugs and immunotherapies. Also, we plan to continue our longstanding and fruitful collaborations with partners providing the required additional expertise (most importantly with research groups in the Applied Molecular Imaging @ Erasmus MC (AIMIE) Core Facility, in the Medical Delta with Delft and Leiden University, in Nijmegen, in Athens, in Montpellier, in the US and with multiple industry partners, including with Novartis/AAA and MILabs B.V.
Preclinical and clinical molecular imaging will continue to play an important role in the preclinical evaluation of these radiotracers as well as in the (back)translational studies that we perform to bring new compounds and techniques into the clinic to improve current compounds and techniques already clinically applied.