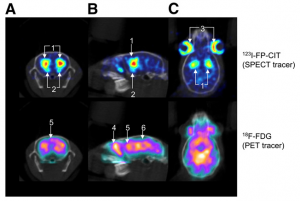
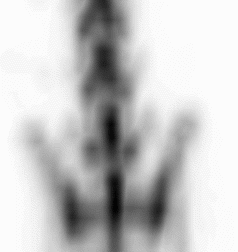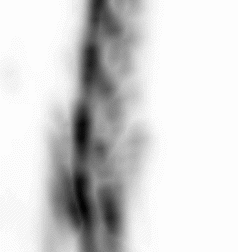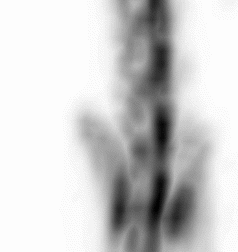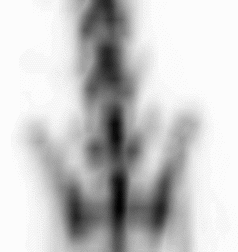Small Animal Brain SPECT-PET
Unique capabilities of MILabs’ U-SPECT and VECTor systems include the quantification of fast dynamics of both SPECT and PET tracers, due to the absence of rotating detectors. VECTor’s ability to acquire simultaneously sub-mm resolution images of multiple SPECT and PET tracers is a unique benefit for researchers and simple to carry out.
Selected publications:
| ► | High-resolution quantitative PET with minimal partial volume effects is essential for brain imaging. In a recent paper by Walker and colleagues (JNM 2014) the importance of high-resolution and high contrast brain imaging using clustered-pinholes was shown and compared with traditional coincidence PET. | |||||||||||||
|
|
|||||||||||||
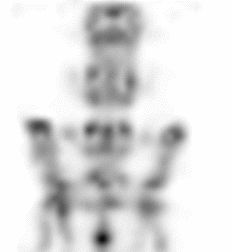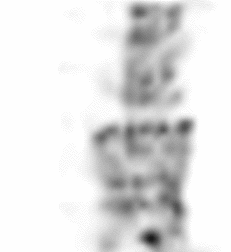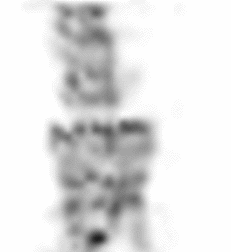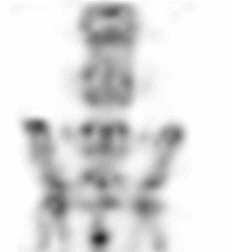
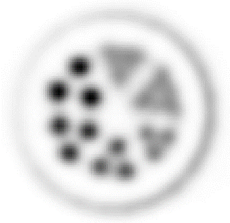
| Images of Jaszczak phantom with rod diameters of 0.85, 0.95, 1.10, 1.30, 1.50, and 1.70 mm. (A) Results for VECTor, (B) Results for coincidence PET using 3DMAP (β=0), for equal duration frames, (C) 18F-NaF bone imaging in pelvis and lower spine of mice. with VECTor and (D) coincidence PET using 3DMap (β=0), (E) 18F-FDG image acquired on VECTor and F. corresponding ex vivo autoradiography of mouse brain [1]. | ||||||||||||||
References
| 1. | Performance Assessment of a Preclinical PET Scanner with Pinhole Collimation by Comparison to a Coincidence-Based Small-Animal PET Scanner, Walker et al. Journal of Nuclear Medicine, p1368-1374, 2014 |
| 2. | Small-animal single-photon emission computed tomographic imaging of the brain serotoninergic systems in wild-type and mdr1a knockout rats. Dumas et al. Molecular Imaging, p1-12, 2014 |
| 3. | Hippocampal deep brain stimulation induces decreased rCBF in the hippocampal formation of the rat, Wyckhuys et al. NeuroImage p55-61, 2010 |
| 4. | VECTor: A Preclinical Imaging System for Simultaneous Submillimeter SPECT and PET, Goorden et al. Journal of Nuclear Medicine, p306-312, 2012 |