Hexamodal Radiomics
Preclinical Tumor Radiomics: Many features – One solution
Comprehensive sampling of the tumor microenvironment, both in space and in time, is expected to have a major impact on cancer research, diagnosis and treatment planning. This so-called radiomics research approach requires the high-throughput extraction of large numbers of in vivo image-based features from tumors to correlate therapy response to genomic and proteomic patterns.
Quantifying the spatial and temporal heterogeneity of small tumors in the preclinical setting puts hereto unmet demands on imaging systems. With the introduction of its scalable Hexamodal systems, MILabs is now offering oncology researchers a unique tool to meet the multi-facetted demands of radiomics imaging. Featuring high-performance Hexamodal imaging technologies, i.e. SPECT, PET, CT, Bioluminescence, Fluorescence and Cherenkov imaging, MILabs enables to extract with just one imaging study, more radiomics information than can be obtained from using multiple preclinical imaging systems:
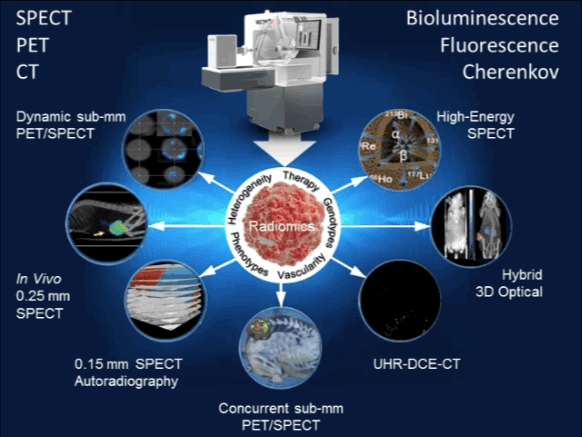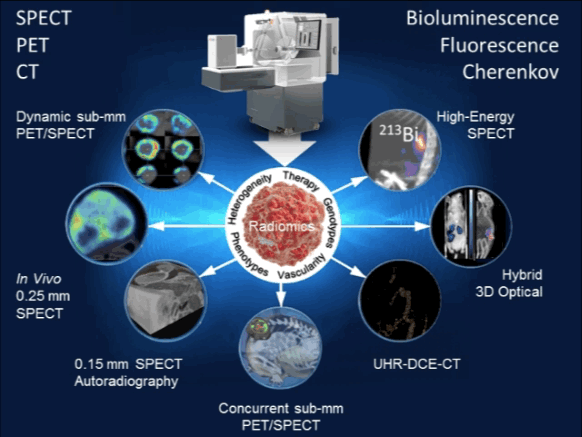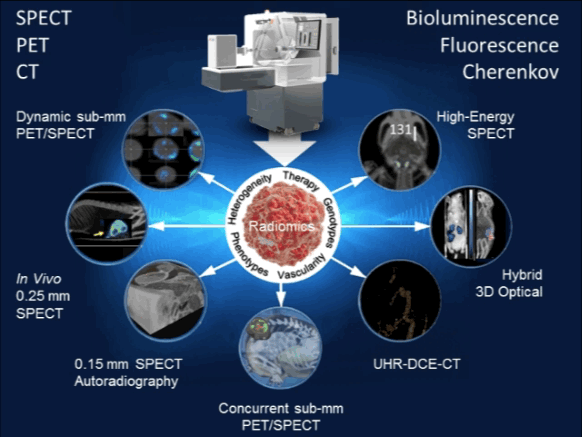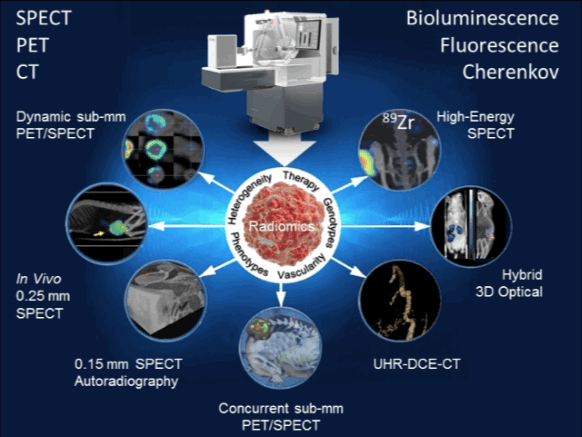 |
| MILabs’ Hexamodal preclinical imaging platform for rapid, multi-feature determination of tumor radiomics. |
Heterogeneity Quantification
Tumors are spatially and temporally heterogeneous. MILabs’ preclinical imaging systems offer the unprecedented ability to dynamically image tumors with SPECT and PET at sub-mm resolutions, even down to 0.15 mm resolution for SPECT. This unique ability enables in vivo quantification of physiological or biological factors that influence tumor response [1–4].
Phenotyping
The physical tumor microenvironment contributes significantly to carcinogenesis, cancer progression and metastatic dissemination. With MILabs’s exclusive 3D SPECT/CT autoradiography technique, the labor-intensive and time-consuming method of serial section and traditional immuno-histochemical staining for protein profiling is becoming a thing of the past. Moreover, for in vivo applications, its multi-parametric simultaneous PET/SPECT imaging of co-injected tracers enables to uncover novel imaging characteristics linked to tumor phenotypes [5,6].
Tumor Vasculature
Factors that impede drug delivery include the complicated, tortuous nature of tumor vasculature. The MILabs’s ultra-high resolution Dynamic Contrast-Enhanced CT (DCE-CT) feature makes it possible to analyze temporal dynamic changes in perfusion parameters in tumor tissue. In addition, simultaneous imaging of vascularity and hypoxia with Concurrent PET/SPECT can differentiate hypervascular from hypovascular tumors, thus guiding the decision for applying antiangiogenic, anti-vascular, or anti-hypoxia therapies [7].
Radiogenomic Genotypes
Radiogenomics analysis reveals gene-expression patterns which often underlay prognostic radiomic signatures, hence providing further insight into intra-tumoral heterogeneity. MILabs’s Hybrid Optical sub-system imaging capability enables imaging of multi-modal reporter gene/reporter probe system thus allowing one to select an appropriate imaging modality for a particular application, cross-validate information captured by each imaging modality, and facilitate translation of preclinical imaging techniques to the clinic [7,8] http://www.milabs.com/contact/download-brochure/.
Radiotherapy Profiling
Targeted radionuclide therapy is one of the most intensively developing directions of nuclear medicine. MILabs’ offers the most versatile preclinical imaging system ever for evaluating strategies to improve radiotherapy, all with the aim of increasing the effect on the tumor or decreasing the effects on normal tissues. Virtually any α-, β-, or Auger electron-emitter with gamma-rays below 600 keV can be imaged with the system and its therapeutic effects quantified. Now you can tailor your irradiation therapy to tumor type and heterogeneity, as well as the inhomogeneity of the radionuclide distribution, pharmacokinetics, etc… No longer will your imaging system limit your options [9–12].
References
| 1. | Miest TS, Frenzke M, Cattaneo R. Measles virus entry through the signaling lymphocyte activation molecule governs efficacy of mantle cell lymphoma radiovirotherapy. Mol Ther. 2013 Nov;21(11):2019–31. Direct link |
| 2. | Shao G, Zhou Y, Wang F, Liu S. Monitoring glioma growth and tumor necrosis with the U-SPECT-II/CT scanner by targeting integrin αvβ3. Mol Imaging. 2013;12(1):39–48. Pubmed link |
| 3. | Branderhorst W, Blezer E LA, Houtkamp M, Ramakers RM, van den Brakel JH, Witteveen H, et al. Three-dimensional histologic validation of high-resolution SPECT of antibody distributions within xenografts. J Nucl Med. 2014 May;55(5):830–7. Direct Link |
| 4. | Ivashchenko O, van der Have F, Villena JL, Groen HC, Ramakers RM, Weinans HH, et al. Quarter-Millimeter-Resolution Molecular Mouse Imaging with U-SPECT+. Mol Imaging. 2014 Nov 1;13:1–8. Pubmed Link |
| 5. | Adachi N, Yoshii Y, Furukawa T, Yoshimoto M, Takeuchi Y, Inubushi M, et al. In Vivo Simultaneous Imaging of Vascular Pool and Hypoxia with a HT-29 Tumor Model: the Application of Dual-Isotope SPECT/PET/CT. International Journal of Sciences: Basic and Applied Research (IJSBAR). 2016. p. 26–39. Direct Link |
| 6. | Miwa K, Inubushi M, Takeuchi Y, Katafuchi T, Koizumi M, Saga T, et al. Performance characteristics of a novel clustered multi-pinhole technology for simultaneous high-resolution SPECT/PET. Ann Nucl Med. 2015 Jun 19;29(5):460–6. Direct Link |
| 7. | Image courtesy of Dr Sean Smart and Dr Veerle Kersemans, CRUK/MRC Oxford Institute for Radiation Oncology, University of Oxford |
| 8. | van Oosterom MN, Kreuger R, Buckle T, Mahn W a, Bunschoten A, Josephson L, et al. U-SPECT-BioFluo: an integrated radionuclide, bioluminescence, and fluorescence imaging platform. EJNMMI Res. 2014 Jan;4:56. Pubmed Link |
| 9. | Suksanpaisan L, Pham L, McIvor S, Russell SJ, Peng K-W. Oral contrast enhances the resolution of in-life NIS reporter gene imaging. Cancer Gene Ther. Nature Publishing Group; 2013 Nov;20(11):638–41. Pubmed Link |
| 10. | ter Heine R, Lange R, Breukels OB, Bloemendal HJ, Rummenie RG, Wakker AM, et al. Bench to bedside development of GMP grade Rhenium-188-HEDP, a radiopharmaceutical for targeted treatment of painful bone metastases. Int J Pharm. Elsevier B.V.; 2014 Apr 25;465(1–2):317–24. Pubmed Link |
| 11. | Esquinas PL, Rodríguez-Rodríguez C, Carlos De La Vega J, Bokharaei M, Saatchi K, Shirmohammad M, et al. (188)Re image performance assessment using small animal multi-pinhole SPECT/PET/CT system. Phys medica. 2016 Dec 19; PubMed Link |
| 12. | de Swart J, Chan HS, Goorden MC, Morgenstern A, Bruchertseifer F, Beekman FJ, et al. Utilizing High-Energy γ-Photons for High-Resolution 213Bi SPECT in Mice. J Nucl Med. 2016 Mar;57(3):486–92. Pubmed Link |
| 13. | van der Have F, Ivashchenko O, Goorden MC, Ramakers RM, Beekman FJ. High-resolution clustered pinhole (131)Iodine SPECT imaging in mice. Nucl Med Biol. 2016 Aug;43(8):50 Pubmed Link |
